Brain Disorders
CNL Research
Multiple Sclerosis
Axons have received less attention than dendrites but are equally important for neural function. When axonal conduction is disrupted, as occurs in demyelinating diseases such as multiple sclerosis (MS), symptoms are diverse according to where the central demyelination occurs and can be either negative (numbness, neuropathy) or positive (tingling, pain). We developed a Hodgkin-Huxley model of a myelinated axon to explore possible explanations for these symptoms and found that the key parameter was the ratio of sodium conductance to potassium leak conductance (Coggan, Prescott et al. 2010). If this ratio was too low, conduction block occurred, but if it was too high, spontaneous action potentials were generated. Most of the effort to find drug treatments for MS has focused on the sodium channel, but our model suggests that the leak conductance may be as important as the sodium conductance. This could lead to a new class of drugs to treat the symptoms of MS.
Epilepsy
During spike-wave seizures, neocortical paroxysmal activity exhibits slow transitions between fast runs (tonic spiking) and slow bursting. The transition takes place over tens of seconds, a time scale that is an order of magnitude longer than the intrinsic dynamics of cortical neurons. We showed in a computational model of a neocortical circuit with extracellular potassium dynamics that activity-dependent modulation of intrinsic excitability can lead to sustained oscillations with slow transitions between fast runs and slow bursting (Frohlich, Bazhenov et al. 2006; Frohlich, Bazhenov et al. 2008; Frohlich, Sejnowski et al. 2010). We predicted that extracellular potassium dynamics can cause alternating episodes of fast and slow oscillatory states in both normal and epileptic neocortical networks (Frohlich, Bazhenov et al. 2008; Frohlich, Bazhenov et al. 2008), reviving a mechanism that had been previously considered as cause of epilepsy but without an understanding of how it might affect the dynamics of paroxysmal discharges (Frohlich, Bazhenov et al. 2008).
We are collaborating with Syd Cash at MGH on recordings from human cortex. In addition to analyzing cortical recordings during sleep, we are also analyzing recordings during seizures. We have had excellent results fitting the raw recordings using delayed differential equations. This method provides an objective way to quickly find the focus of the seizure and has revealed a sequence of substates that occur before, during and after the seizures. The model is surprisingly simple, having only 3 terms with fixed time delays. The 3 parameters are fit to one second windows of data and the error is tracked over time. The reason why this relatively simple model can fit brain recordings so well will be explored using cortical models of epilepsy (Frohlich, Bazhenov et al. 2006; Frohlich, Bazhenov et al. 2008; Frohlich, Sejnowski et al. 2010).
Schizophrenia
Cortical circuits have approximately balanced excitatory and inhibitory activity with membrane potentials near thresholds. The central hypothesis that has emerged from both experiments and models (Salinas and Sejnowski 2001; Haider 2010) is that in a balanced condition, a neural circuit can rapidly organize the spike timing of activated neurons into synchronous events. Synchrony is a mechanism for rapidly changing the gain of a neural circuit (Wang, Spencer et al. 2010). My lab has focused on the fast-spiking, parvalbumin positive (FS-PV) cortical basket cells (Hasenstaub, Otte et al. 2010), which are implicated in generating gamma band oscillations (Tiesinga and Sejnowski 2009).
FS- PV cortical basket cells can be pharmacologically lesioned from the circuit by injections of subanaesthetic does of ketamine, an NMDA channel blocker, on two successive days (Behrens and Sejnowski 2009). In the adult, the GAD67 and PV genes are downregulated for a week following the injections, but we have recently shown that when injected during the second postnatal week, the downregulation is permanent, as shown in Fig. 6 (Pinto-Duarte 2010). We will study the intrinsic properties of the FS-PV interneurons in cortical slices from a range of cortical areas and stimulus-induced gamma in the EEG of ketamine treated mice.
We have shown that blocking DNA methylation in the neonate mouse blocks the ketamine induced downregulation of GAD67 and PV (Behrens 2010). This suggests a failure of these cells to complete their normal development. We are collaborating with Joe Ecker at Salk to sequence the methylome of the FS-PV interneurons during early development and in the ketamine-treated mouse to search for genes that are abnormally methylated. The ketamine mouse has some of the biological and behavioral markers of schizophrenia, such as impaired pre-pulse inhibition, learning deficits and a decrease in stimulus-induced gamma. Although GAD67 is downregulated, the FS-PV neurons are still alive (Fig. 6). A long-term goal is to reverse the methylation pattern induced by ketamine and return the circuit to its normal function.
An inhibition-stabilized network model of a cortical column (Tsodyks, Skaggs et al. 1997; Ozeki 2009) will be used to explore the effects of stimulus contrast and surround effects on the frequency and power of the gamma oscillation (Jadi 2011). The impact of lesioning the feedback inhibition on the oscillations will be examined and compared to the results of the ketamine treated mice. This model will be extended to a 2D array of neurons to explore the spatial coherence of the oscillations.
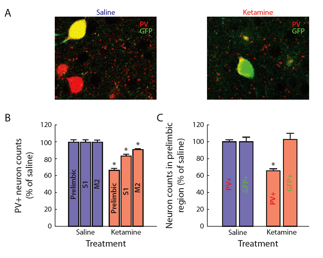
Fig. 1 Perinatal exposure to ketamine reduces the number of PV-expressing interneurons in the adult frontal cortex. G42 mice expressing GFP in FS-PV cells were injected with either saline or ketamine on postnatal days 7, 9, and 11. When they reached 8 weeks of age, their brains were immunostained for parvalbumin. All cells in the region were counted and normalized to the saline samples. (Powell, Sejnowski et al. 2011)
References:
- Behrens, M., Hasenstaub, A., Sejnowski, T.J. (2010). "A role for DNA methylation in the NMDA receptor antagonist-mediated loss of phenotype of parvalbumin-positive fast-spiking interneurons." Society for Neuroscience Abstracts.
- Behrens, M. M. and T. J. Sejnowski (2009). "Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex?" Neuropharmacology 57(3): 193-200.
- Coggan, J. S., S. A. Prescott, et al. (2010). "Imbalance of ionic conductances contributes to diverse symptoms of demyelination." Proceedings of the National Academy of Sciences of the United States of America 107(48): 20602-20609.
- Frohlich, F., M. Bazhenov, et al. (2008). "Potassium dynamics in the epileptic cortex: new insights on an old topic." The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 14(5): 422-433.
- Frohlich, F., M. Bazhenov, et al. (2008). "Pathological effect of homeostatic synaptic scaling on network dynamics in diseases of the cortex." The Journal of neuroscience : the official journal of the Society for Neuroscience 28(7): 1709-1720.
- Frohlich, F., M. Bazhenov, et al. (2006). "Slow state transitions of sustained neural oscillations by activity-dependent modulation of intrinsic excitability." The Journal of neuroscience : the official journal of the Society for Neuroscience 26(23): 6153-6162.
- Frohlich, F., T. J. Sejnowski, et al. (2010). "Network bistability mediates spontaneous transitions between normal and pathological brain states." The Journal of neuroscience : the official journal of the Society for Neuroscience 30(32): 10734-10743.
- Haider, B., Krause, M.R., Duque, A., Yu, Y., Touryan, J., Mazer, J.A., McCormick, D.A. (2010). "Synaptic and network mechanisms of sparse and reliable visual cortical activity during nonclassical receptive field stimulation." Neuron 65: 107-121.
- Hasenstaub, A., S. Otte, et al. (2010). "Metabolic cost as a unifying principle governing neuronal biophysics." Proceedings of the National Academy of Sciences of the United States of America 107(27): 12329-12334.
- Jadi, M. P. S., T. J. (2011). "Inhibition stabilized model of cortical network & contrast dependence of cortical rhythms in the visual cortex." Cosyne.
- Ozeki, H., Finn, I.M., Schaffer, E.S., Miller K.D. and Ferster, D. (2009). "Inhibitory stabilization of the cortical network underlies visual surround suppression." Neuron 62: 578-592.
- Pinto-Duarte, A., Bonjean, M., Behrens, M.M., Sejnowsi, T.J. (2010). "Neonatal exposure to NMDA receptor antagonists halts the maturation of parvalbumin-positive fast-spiking interneurons leading to altered network activity in adulthood." Society for Neuroscience Abstracts.
- Powell, S. B., T. J. Sejnowski, et al. (2011). "Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia." Neuropharmacology.
- Salinas, E. and T. J. Sejnowski (2001). "Correlated neuronal activity and the flow of neural information." Nature reviews. Neuroscience 2(8): 539-550.
- Tiesinga, P. and T. J. Sejnowski (2009). "Cortical enlightenment: are attentional gamma oscillations driven by ING or PING?" Neuron 63(6): 727-732.
- Tsodyks, M. V., W. E. Skaggs, et al. (1997). "Paradoxical effects of external modulation of inhibitory interneurons." The Journal of neuroscience : the official journal of the Society for Neuroscience 17(11): 4382-4388.
- Wang, H. P., D. Spencer, et al. (2010). "Synchrony of thalamocortical inputs maximizes cortical reliability." Science 328(5974): 106-109.
